Lab Publication - Cancer Genetics
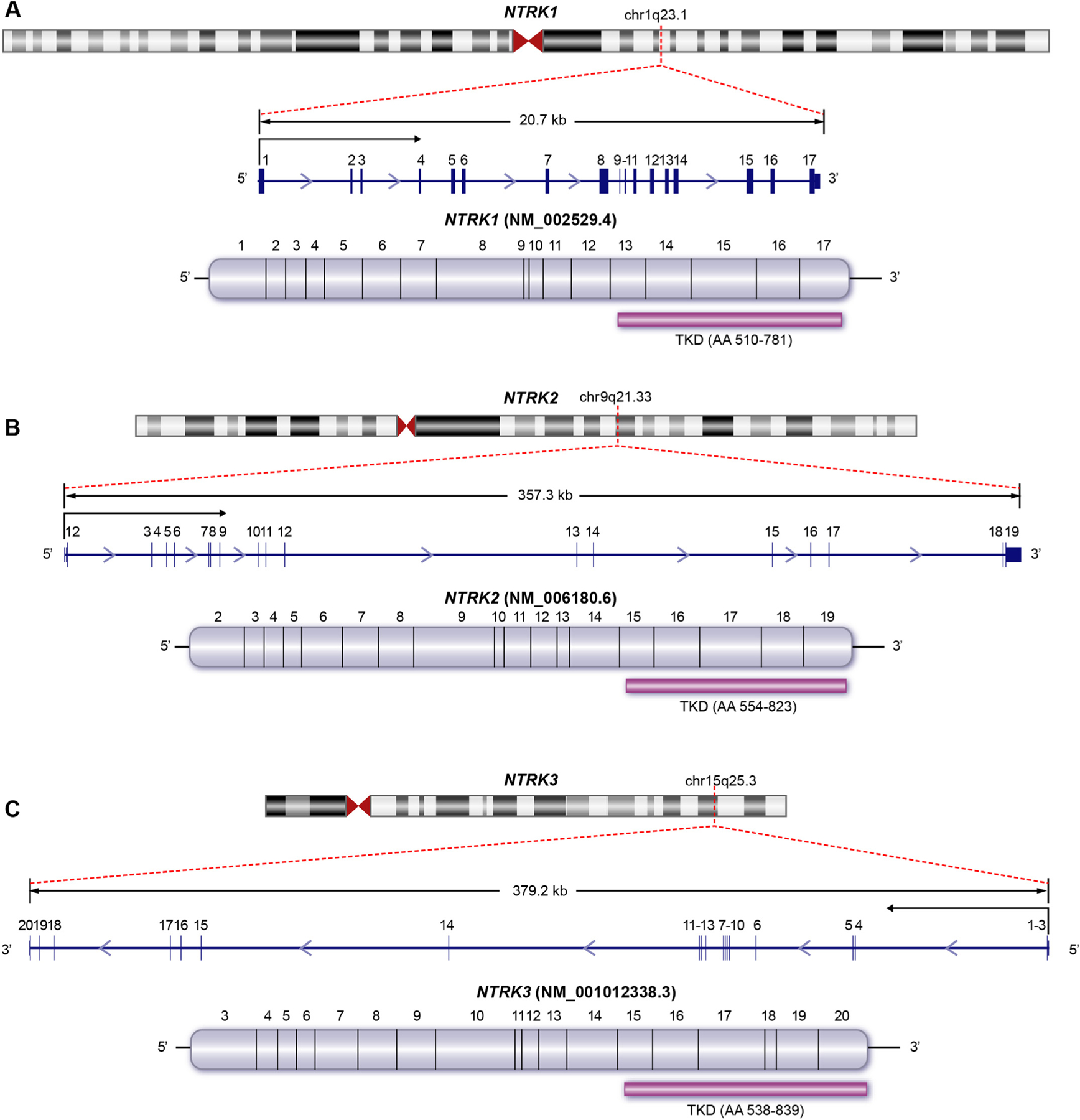
Gene fusions involving the neurotrophic receptor tyrosine kinase genes NTRK1, NTRK2, and NTRK3, are well established oncogenic drivers in a broad range of pediatric and adult tumors. These fusions are also important actionable markers, predicting often dramatic response to FDA approved kinase inhibitors. Accurate interpretation of the clinical significance of NTRK fusions is a high priority for diagnostic laboratories, but remains challenging and time consuming given the rapid pace of new data accumulation, the diversity of fusion partners and tumor types, and heterogeneous and incomplete information in variant databases and knowledgebases. The ClinGen NTRK Fusions Somatic Cancer Variant Curation Expert Panel (SC-VCEP) was formed to systematically address these challenges and create an expert-curated resource to support clinicians, researchers, patients and their families in making accurate interpretations and informed treatment decisions for NTRK fusion-driven tumors. We describe a system for NTRK fusion interpretation (including compilation of key elements and annotations) developed by the NTRK fusions SC-VCEP. We illustrate this stepwise process on examples of LMNA::NTRK1 and KANK1::NTRK2 fusions. Finally, we provide detailed analysis of current representation of NTRK fusions in public fusion databases and the CIViC knowledgebase, performed by the NTRK fusions SC-VCEP to determine existing gaps and prioritize future curation activities.
Read the paper: Cancer Genetics
